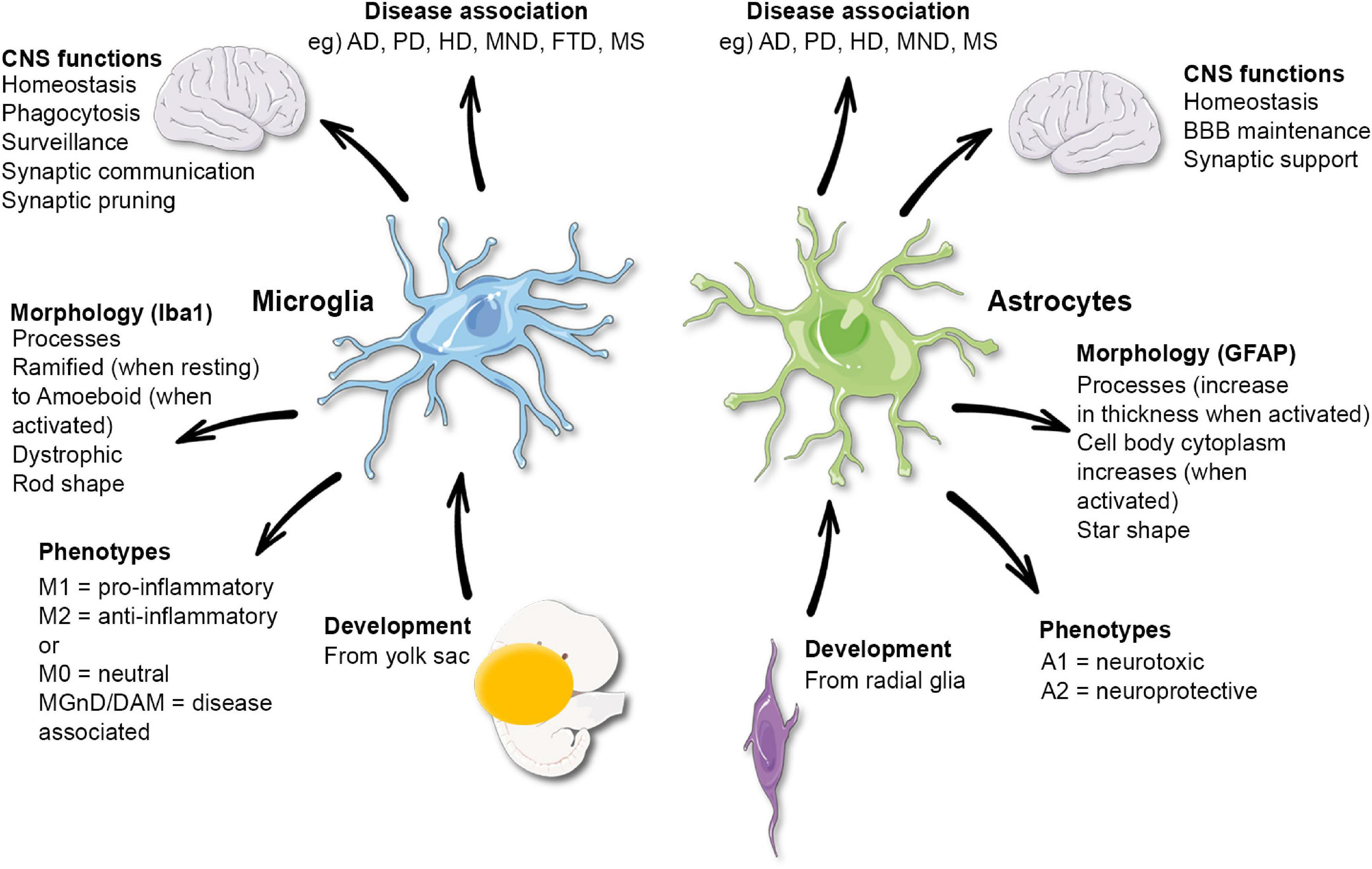
Microglial research is at the forefront of scientific inquiry aimed at understanding the brain’s immune system and its role in neurodegenerative disorders such as Alzheimer’s disease. These remarkable cells are crucial in maintaining the health of the brain, acting as sentinels that monitor for damage and clear away debris, while also engaging in the process of synaptic pruning. Recent discoveries from renowned scientists like Beth Stevens have shed light on how dysfunctional microglial activity can exacerbate conditions like Alzheimer’s, highlighting their dual role in protecting and potentially harming neuronal health. Through rigorous exploration and innovative techniques, this area of research is paving the way for developing new biomarkers and therapeutic strategies geared towards combating the millions affected by neurodegenerative diseases. By unraveling the complexities of microglial function, we stand on the brink of breakthroughs that could revolutionize our approach to brain health and disease management.
The study of glial cells, particularly microglia, offers profound insights into the immune mechanisms within the brain and their implications for conditions involving cognitive decline. These brain-resident immune cells play a crucial role not just in responding to injury but also in shaping neural circuits through synaptic remodeling. Researchers, such as Beth Stevens, have significantly contributed to our understanding of how microglial behavior can become skewed in diseases like Alzheimer’s and other neurodegenerative disorders, calling attention to their importance in the brain’s overall well-being. By investigating these cellular dynamics, scientists are uncovering potential pathways that may ultimately lead to effective treatments and prevention strategies for those grappling with Alzheimer’s disease. Thus, glial cell research stands as a vital avenue for discovering how we might protect and repair the brain against various cognitive impairments.
Understanding Microglial Cells in Neurodegenerative Disorders
Microglial cells play a crucial role in the brain’s immune response, acting as the first line of defense against neurodegenerative disorders like Alzheimer’s disease. These specialized cells continuously survey the brain environment, identifying and clearing out dead neurons and debris that could threaten cognitive function. Their ability to prune synapses—connections between neurons—is vital during normal brain development, as it ensures that only the most efficient neural pathways are maintained. However, when this process goes awry, it may contribute to various neurodegenerative conditions, highlighting the dual nature of microglia as both protectors and potential aggressors in brain health.
Research conducted by Beth Stevens and her team has provided groundbreaking insights into how microglial dysfunction can be linked to diseases such as Alzheimer’s and Huntington’s disease. By examining the mechanisms of synaptic pruning in both healthy and diseased brain states, Stevens’ lab has shed light on the biological processes that may lead to increased neuroinflammation and neuronal damage. This understanding opens new channels for developing targeted therapies that can modulate microglial activity to prevent or mitigate these neurodegenerative diseases.
The Role of Synaptic Pruning in Alzheimer’s Disease
Synaptic pruning, a process regulated by microglia, plays an essential role in maintaining healthy brain function. In the context of Alzheimer’s disease, aberrant synaptic pruning can lead to significant cognitive decline, as the loss of crucial synapses disrupts communication among neurons. Stevens’ research has revealed that when microglia over-prune synapses, it may result in the accelerated progression of Alzheimer’s pathology. Understanding this mechanism is critical, as it provides a potential therapeutic target to restore normal pruning functions and protect against synapse loss.
Additionally, Stevens emphasizes that not all synaptic pruning is harmful. Normal pruning is a necessary part of neural development, shaping the brain’s circuitry during critical growth periods. However, the challenge lies in distinguishing between healthy and pathological pruning processes. Through continued exploration of these mechanisms, researchers hope to identify biomarkers that can predict the onset of Alzheimer’s disease, paving the way for early intervention and better management of this devastating condition.
The Impact of Basic Science on Neurodegenerative Research
Basic science research is the cornerstone upon which transformative medical advancements are built. In the case of neurodegenerative disorders, fundamental studies into the biology of microglia have illuminated their multifaceted roles in brain health. As Stevens points out, much of the progress made in understanding disease mechanisms stems from curiosity-driven research that initially may seem removed from direct clinical applications. This type of inquiry not only expands our knowledge base but also fosters innovation, leading to promising new treatments for Alzheimer’s and similar diseases.
Stevens’ extensive work exemplifies how foundational research, supported by institutions like the NIH, contributes to a better understanding of the brain’s immune system. By building insights from animal models, scientists can explore complex interactions that are difficult to replicate in human studies. This foundational knowledge ultimately enhances our ability to design effective strategies for disease prevention and treatment, reminding us of the profound importance of supporting basic scientific research in paving the way for future breakthroughs.
Funding and Support in Neuroglial Research
The journey of scientific discovery is often unpredictable, driven by the need for substantial funding and support from government agencies. In her work on microglial research, Beth Stevens highlights the vital role of funding from the National Institutes of Health in advancing our understanding of Alzheimer’s disease and other neurodegenerative disorders. This funding enables researchers to pursue innovative avenues of inquiry without immediate pressure for clinical outcomes, fostering an environment where groundbreaking ideas can flourish.
Stevens emphasizes that federal grants have been pivotal in her lab’s ability to explore complex questions about the brain’s immune system. The support not only provides financial resources but also validates the importance of scientific inquiry. By investing in basic research, we can unlock the potential for new discoveries that may lead to effective treatments for conditions like Alzheimer’s disease, ultimately improving lives and enhancing our understanding of the human brain.
Future Directions in Microglial Research
As we look to the future of microglial research, the potential for groundbreaking discoveries continues to expand. Recent advancements in technology, such as high-resolution imaging and genetic editing tools like CRISPR, allow scientists to study microglial function in unprecedented detail. Understanding the interactions between microglia and neurons during the progression of neurodegenerative diseases could lead to the identification of new therapeutic targets and biomarkers, offering a glimmer of hope for millions affected by conditions like Alzheimer’s.
Furthermore, as research continues to elucidate the complexities of the brain’s immune system, interdisciplinary collaborations among neurologists, immunologists, and geneticists can drive comprehensive investigations into the role of microglia. By leveraging diverse expertise, researchers have the opportunity to uncover the underlying mechanisms of neuroinflammation and synaptic dysfunction, ultimately contributing to the development of innovative strategies to combat neurodegenerative disorders affecting an ever-increasing population.
Neuroinflammation: A Common Thread in Neurodegenerative Disorders
Neuroinflammation is increasingly recognized as a central player in various neurodegenerative disorders, including Alzheimer’s disease and multiple sclerosis. Microglia, the brain’s primary immune cells, become activated in response to cellular stress and damage, leading to inflammation that can exacerbate neuronal injury. In the context of Alzheimer’s, chronic neuroinflammation often correlates with cognitive decline, highlighting the importance of understanding how microglial activation is regulated. Research efforts aimed at unraveling these complexities offer promising avenues for therapeutic interventions targeting inflammation.
As studies continue to explore the relationship between neuroinflammation and synaptic health, it becomes clear that modulating microglial activity may hold the key to restoring balance in the brain. By addressing neuroinflammation early in the disease process, it may be possible to reduce synaptic loss and improve cognitive outcomes for patients with Alzheimer’s disease. This highlights the need for ongoing research into the mechanisms by which microglia contribute to both neuroprotection and neurodegeneration.
The Innovative Approaches of the Stevens Lab
The Stevens Lab stands at the forefront of neuroimmune research, employing innovative approaches to unravel the complexities of microglial function in neurodegenerative diseases. By integrating cutting-edge techniques such as single-cell RNA sequencing, researchers can assess the unique molecular profiles of microglia and their responses to various stimuli within the brain environment. This approach not only provides insights into the functional diversity of microglial cells but also helps identify specific pathways that could be targeted for therapeutic purposes.
Moreover, the collaborative nature of the Stevens Lab facilitates a rich exchange of ideas between scientists from diverse backgrounds, fostering a collegial atmosphere that emphasizes creativity and discovery. This multidisciplinary environment allows for the exploration of complex interactions between microglia and other cell types, ultimately leading to a more comprehensive understanding of neurodegenerative processes. Through these innovative research strategies, the lab is paving the way for the development of new interventions that could significantly alter the trajectories of diseases like Alzheimer’s.
The Significance of Biomarkers in Alzheimer’s Research
Identifying reliable biomarkers is crucial for early diagnosis and treatment of Alzheimer’s disease. Biomarkers can provide valuable information regarding disease progression and severity, thus allowing for timely intervention strategies. The research conducted by Beth Stevens and her team emphasizes the role of microglial activity and synaptic health as potential biomarkers for Alzheimer’s. As our understanding of how microglia interact with neurons continues to evolve, these biomarkers could facilitate earlier detection and better-targeted therapies, improving patient outcomes.
Furthermore, the incorporation of imaging techniques and fluid-based biomarkers may enhance diagnostic precision. Innovative approaches such as PET imaging can help visualize the presence of amyloid plaques and tau protein tangles in the brain, but emerging studies suggest that measuring microglial activation may provide complementary insights. By integrating multiple biomarker strategies, researchers can create a comprehensive profile of Alzheimer’s disease, supporting personalized treatment approaches that target the distinct pathologies affecting each patient.
Conclusion: The Future of Microglial Research and Alzheimer’s Disease
The future of microglial research holds tremendous promise for advancing our understanding and treatment of Alzheimer’s disease and other neurodegenerative disorders. As the field continues to grow, collaboration between researchers, clinicians, and funding organizations will be essential to tackle the ongoing challenges posed by these complex conditions. This collective effort is crucial for translating basic scientific discoveries into tangible clinical applications that can aid millions affected by neurodegenerative diseases.
Ultimately, continued investment in innovative research focused on the brain’s immune system, led by pioneers like Beth Stevens, will be instrumental in unlocking the mysteries of Alzheimer’s disease. With the advancement of technologies and interdisciplinary approaches, we may finally unlock new pathways for intervention that lead to effective therapies, improved quality of life, and hope for those impacted by these debilitating conditions.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease and neurodegenerative disorders?
Microglial cells serve as the brain’s immune system, patrolling for signs of illness or injury. In Alzheimer’s disease and other neurodegenerative disorders, they help clear away dead or damaged cells and are involved in synaptic pruning, which can, when functioning aberrantly, contribute to disease progression.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is a prominent neuroscientist and NIH-supported investigator who has significantly advanced our understanding of microglial cells and their role in Alzheimer’s disease. Her research has revealed how microglia can contribute to synaptic pruning and disease mechanisms in neurodegenerative disorders.
How does microglial research inform new treatments for Alzheimer’s disease?
By studying microglial cells and their functions, researchers like Beth Stevens are identifying new biomarkers and potential therapies for Alzheimer’s disease. Understanding the immune response and synaptic pruning processes can lead to innovative treatment strategies that address the underlying causes of neurodegenerative disorders.
What is synaptic pruning and how is it related to microglial cells?
Synaptic pruning is the process by which unnecessary or excess synapses are eliminated, facilitating efficient neural connections. Microglial cells are essential in this process, as they help sculpt brain circuitry during development and maintain neuronal health, but their dysfunction can lead to issues in Alzheimer’s disease and other neurodegenerative disorders.
Why is Beth Stevens’ microglial research considered foundational for understanding Alzheimer’s disease?
Beth Stevens’ microglial research has laid the groundwork for exploring how the brain’s immune system influences neurodegenerative conditions like Alzheimer’s disease. Her findings on the relationship between aberrant microglial pruning and disease pathology have opened new avenues for diagnostic and therapeutic approaches.
How can microglial research impact the care of individuals with Alzheimer’s disease?
Microglial research holds promise for improving the care of the estimated 7 million Americans living with Alzheimer’s disease by contributing to the development of new biomarkers for early detection and targeted therapies, ultimately enhancing patient management and treatment outcomes.
| Key Point | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, monitoring for illness and clearing out damaged cells. |
| Aberrant Pruning | Improper pruning by microglia can contribute to neurodegenerative diseases, including Alzheimer’s and Huntington’s. |
| Research Foundation | Beth Stevens’ research is supported by NIH and focuses on using basic science to inform treatment approaches. |
| Impact on Patients | Research could significantly affect care for approximately 7 million Americans living with Alzheimer’s. |
| Curiosity-Driven Science | Basic science in animal models facilitates understanding of human diseases, contributing to new treatment strategies. |
Summary
Microglial research is pivotal for understanding neurodegenerative diseases and developing potential treatments. As demonstrated by Beth Stevens’ groundbreaking work, microglia play a crucial role in brain health by maintaining synaptic integrity and immune response. This research not only sheds light on the mechanisms of diseases like Alzheimer’s but also opens new avenues for therapeutic exploration, underscoring the importance of federal support in pursuing scientific inquiry. Through a dedication to curiosity-driven research, significant advancements in the field of neurobiology can lead to impactful strategies in combating debilitating conditions.